
Technology features of platform
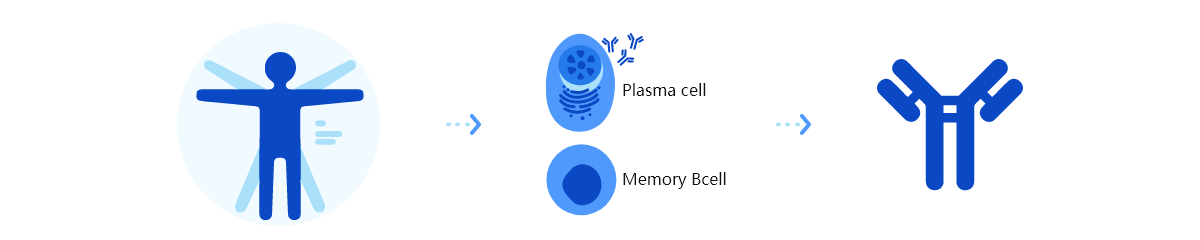
Trinomab has developed world-class fourth-generation of monoclonal antibody (mAb) technology platform (HitmAb?), a proprietary high throughput comprehensive technology platform that integrates the various modern technologies to discover fully native human mAbs through the process of identifying human individuals with desired antibodies for specific application targets, single-cell isolation, single-cell antibody gene amplification, antibody gene sequencing, high throughput antibody gene expression and functional evaluation, with great competitive edge over the other mAb technologies.
Most of currently approved mAbs are derived from mouse-human chimeric or humanized mouse antibodies, or the so-called “fully” human antibodies. Chimeric and humanized antibodies have un-predictable fate in long-time development processes because of high risk of immunogenicity and cross-reactivity that can only be truly assessed in large-scale human clinical trials. The so-called “fully” human antibodies derived from human-Ig gene transgenic mice and/or phage display are misleading in its name and are NOT truly human because these antibodies were not selected through human immune tolerance mechanism and they are likely immunogenic in human.
In comparison, native human mAbs developed by HitmAb faithfully represent in vivo human antibodies that have undergone through selection processes by human immune tolerance mechanism. Therefore, native human mAbs have no or minimal immunogenicity in human and have no or minimal risk to induce anti-drug antibody (ADA) response or off target to cross react with human host antigens. Furthermore, some of the better antibodies can only be developed in and isolated from humans. Thus, native human mAb theapeutics should exhibit better safety and efficacy.
 Technology features of platform
Technology features of platform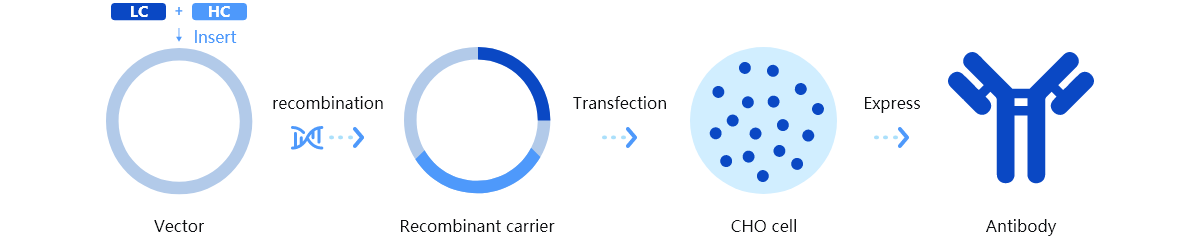
 Technology features of platform
Technology features of platform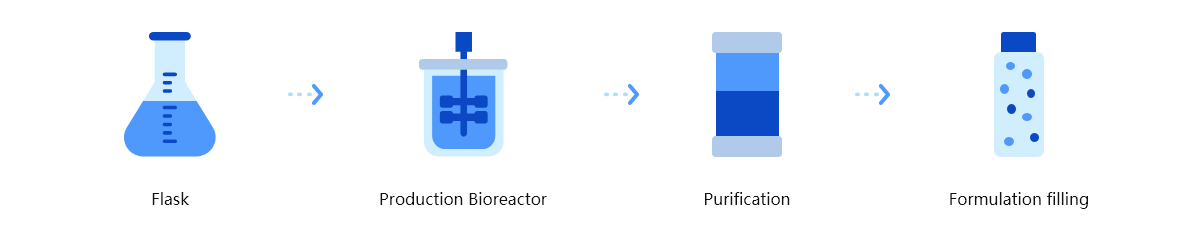
 Technology features of platform
Technology features of platform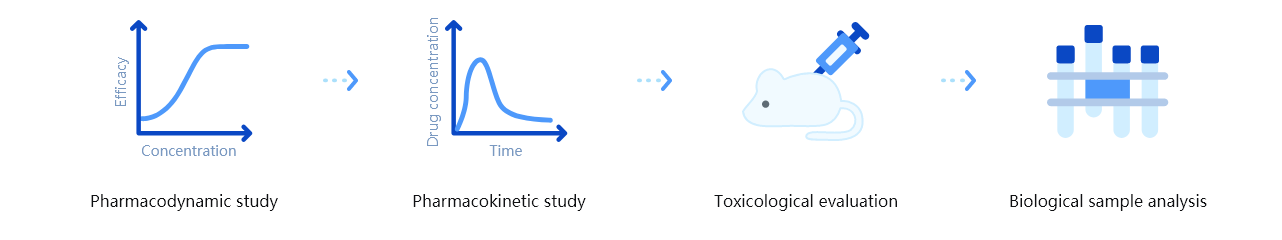
 Technology features of platform
Technology features of platform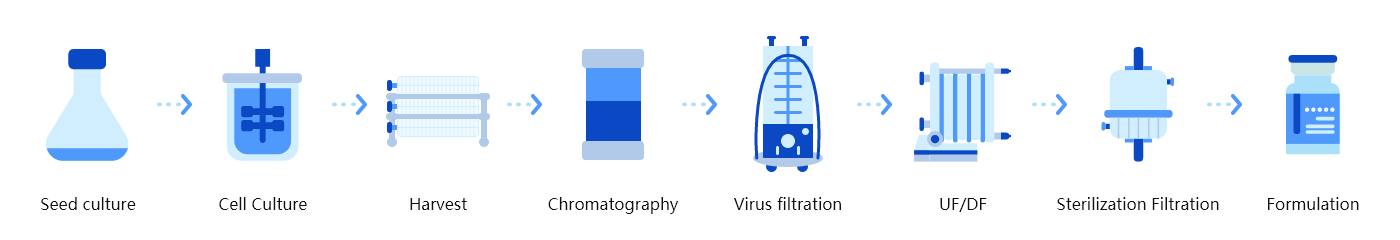
 Technology features of platform
Technology features of platform